Porous imine-based networks with protonated imine linkages for carbon dioxide separation from mixtures with nitrogen and methane†
Abstract
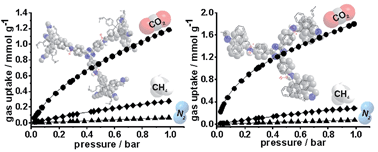
Porous imine-linked networks (PINs) have been prepared by catalyst-free Schiff base condensation of 2,4,6-(4-aminophenyl)1,3,5-triazine with 9,10-anthracene-dicarboxaldehyde (PIN1 and PIN1_2) and with 4,4′,4′′,4′′′-methanetetrakis-benzaldehyde (PIN2). Even after synthesis optimisation, only with DMSO (PIN1 and PIN2) microporous frameworks were obtained, whereas DMF (PIN1_2) and other solvents led to nonporous systems, estimated by argon measurements. Furthermore, 15N solid state NMR and IR spectroscopy reveal the influence of the decomposition products of DMSO leading to protonation of the imine linkage and resulting in an ionic structure with an incorporated sulfonic counterion. Until now, protonation of an imine function due to the usage of DMSO as solvent has not been discussed in the literature. In contrast, for PIN1_2 the imine linkage remains neutral. Argon sorption isotherms for PIN1 and PIN2 exhibit surface areas of 458 m2 g−1 and 325 m2 g−1 and the pore structure displays micro- and small mesopores with pore diameters from 0.6 to 5 nm. Additionally, CO2 isotherms at 273 K demonstrate ultramicropores for all three polymers with similar pore size distributions. Despite their different network structures, similar CO2 uptakes (1.8–1.06 mmol g−1 at 273 K at 1.0 bar) and heat of adsorption Qst values of 30 kJ mol−1 were obtained. In line with the polyionic character of PIN1 and PIN2, both compounds adsorb three times more water than the more hydrophobic, neutral PIN1_2 at room temperature indicating two different adsorption mechanisms. IAST selectivity calculations show good CO2/N2 (30–31) and CO2/CH4 (8–12) selectivity factors. Despite their moderate BET-surface areas, the PINs show good CO2 uptakes and selectivity factors.

 Please wait while we load your content...
Please wait while we load your content...