Ab initio study of sodium intercalation into disordered carbon†
Abstract
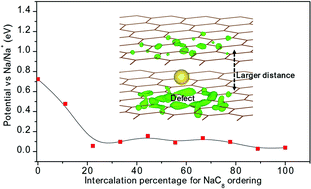
Graphite, a predominantly chosen anode material for commercial lithium ion batteries (LIBs), has been reported to have negligible intercalation capacity as an anode for sodium ion batteries (NIBs). Disordered carbon exhibits high Na intercalation capacity and emerges as a leading candidate for NIB applications. However, the mechanism of Na+ ion insertion into disordered carbon is still controversial. Here, we propose an ab initio model for disordered carbon and investigate the intercalation mechanism of Na into the layered domains. Our ab initio calculations reveal that a larger interlayer distance and the presence of defects can effectively overcome the van der Waals interaction between graphene sheets and help Na intercalation to form NaC8. The calculation results clarify the mechanism of the Na intercalation and account for the presence of sloping and flat regions of charge–discharge curves in disordered carbon reported in numerous experiments. This reveals new prospects for helping Na intercalation into graphite.
- This article is part of the themed collection: 2015 Journal of Materials Chemistry A Hot Papers

 Please wait while we load your content...
Please wait while we load your content...