Carbon-doped porous boron nitride: metal-free adsorbents for sulfur removal from fuels†
Abstract
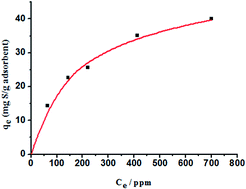
Novel carbon-doped porous boron nitride (C-BN) has been successfully prepared by using 1-butyl-3-methylimidazolium tetrafluoroborate ([Bmim]BF4) as a soft template and the carbon source via calcination under N2 atmosphere. Multiple techniques were applied to investigate the structure, morphology, and adsorptive desulfurization performance. The metal-free porous C-BN displayed enhanced adsorption performance for dibenzothiophene (DBT) than pure BN materials and exhibited one of the highest adsorption capacities reported up to now (49.75 mg S g−1 adsorbent according to the Langmuir isotherm model, 35.2 mg S g−1 adsorbent for 500 ppm sulfur model oil). After three times recycling, the adsorption capacity slightly decreased from 35.2 to 27.2 mg S g−1 adsorbent. The excellent adsorption performance of porous C-BN was attributed to the more exposed atoms along the edges of the pores and the stronger Lewis acid–base interactions between DBT and carbon-doped porous BN. Moreover, it is believed that this strategy to control the structure and composition of BN can be extended to incorporate other heteroatoms and control the pore size for BN materials by changing the anion or cation of the ionic liquids.

 Please wait while we load your content...
Please wait while we load your content...