Nickel nitride as an efficient electrocatalyst for water splitting†
Abstract
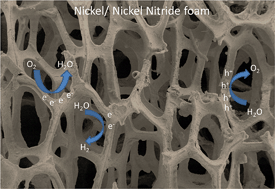
Efficient, robust and low cost materials as electrocatalysts for energy-related applications are highly desired for the future of renewable energy production. Here we show a simple method to fabricate nickel nitride (Ni3N) on nickel (Ni) foam for electrocatalytic applications. The Ni3N/Ni-foam exhibits extremely low overpotential (∼50 mV), high current density and excellent stability for the hydrogen evolution reaction (HER) in alkaline solution. In addition, the modified foam demonstrates enhanced activity in the oxygen evolution (OER) and reduction (ORR) reaction compared to original Ni-foam. The activity enhancement can be attributed to the facile formation of a Ni(OH)2 layer on the nitride layer due to improved lattice matching. The formation of the Ni3N/Ni(OH)2 catalyst results in lower overpotentials due to easier water dissociation on the nickel hydroxide layer. In addition, the HER is further improved due to stronger adsorption of hydrogen to the metal nitride than to the pure metal. We believe that the utilization of nickel nitride as an electrocatalyst opens opportunities for energy-related devices such as batteries and fuel cells.

 Please wait while we load your content...
Please wait while we load your content...