Highly reduced molybdophosphate as a noble-metal-free catalyst for the reduction of chromium using formic acid as a reducing agent†
Abstract
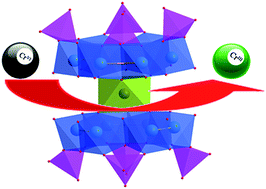
Highly reduced molybdophosphates with the formula (H2bpp)6{Co[Mo6O12(OH)3(HPO4)3-(H2PO4)]2}2·13H2O (1), (H2bpp)6[Co(H2O)2]4{Co[Mo6O12(OH)3(HPO4)2(H2PO4)(PO4)]2}2·2(HPO4)·12H2O (2), [Na(H2O)]2[Co(mbpy)(H2O)3]2[Co(mbpy)(H2O)]2{Co[Mo6O12(OH)3(HPO4)3(PO4)]2}·8H2O (3), and Na2[Co(mbpy)(H2O)3]4{Co[Mo6O12(OH)3(HPO4)3(PO4)]2}·13H2O (4) (bpp = 1,3-bi(4-pyridyl)propane, mbpy = 5,5′-dimethyl-2,2′-dipyridyl) have been synthesized and characterized. The primary structure of an anionic moiety in 1–4 consists of a Co(II) ion bridging two highly reduced [P4Mo6VO28(OH)3]9− ({P4Mo6}) units into an hourglass-shape {Co(P4Mo6)2} cluster. Four supramolecular assemblies have isomers and polymorphs with the difference in organic moieties: flexible bpp for 1 and 2 and chelated mbpy for 3 and 4, revealing that pH values play important roles in the assembling process of hybrids. Electrochemical and catalytic properties of these hybrids are investigated. The preliminary experiments show that this type of hybrid system is active for the electron transfer reaction of chromium(VI) reduction using formic acid at ambient temperature. Experimental results indicate that the reduction reaction can be carried out in a heterogeneous system with a lower reaction temperature and shorter reaction time. This kind of hybrid material is easy to prepare and structurally design, and has the potential to replace precious metals such as Pt/Pd nanoparticles used in this field.

 Please wait while we load your content...
Please wait while we load your content...