Naturally derived porous carbon with selective metal- and/or nitrogen-doping for efficient CO2 capture and oxygen reduction†
Abstract
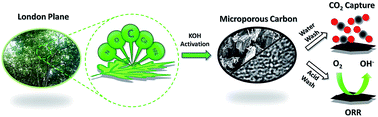
A heterogeneously porous “green carbon” structure was derived from abundant London plane leaves and shows excellent performance for both CO2 capture and Oxygen Reduction Reaction (ORR). The carbonised and KOH-activated carbon possesses a high level of micropores, a specific surface area exceeding 2000 m2 g−1 and a large pore volume of over 1 cm3 g−1, leading to an excellent CO2 uptake of 19.4 wt% under ambient conditions and fast four-electron transfer in an alkaline medium for ORR. Furthermore, XPS and X-ray analyses reveal well-dispersed metal elements (such as Mg and Ca) in the porous carbon, which are naturally doped and inherited from the leaf structure, and can help to enhance CO2 adsorption. On the other hand, these metal elements do not positively affect catalytic ORR performance. Hence, a purpose-specific cleaning approach after KOH activation, i.e. by water or acid, has been devised to obtain optimal functionalities for CO2 capture or ORR.
- This article is part of the themed collection: Highlighting materials research in the UK for energy and sustainability

 Please wait while we load your content...
Please wait while we load your content...