New anhydrous proton exchange membranes for high-temperature fuel cells based on PVDF–PVP blended polymers†
Abstract
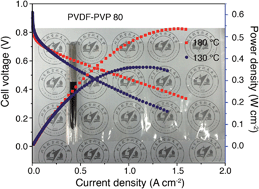
A novel high-temperature proton exchange membrane (PEM) consisting of polyvinylpyrrolidone (PVP) and polyvinylidene fluoride (PVDF) has been successfully prepared by a simple and scalable polymer blending method. PVP is miscible with PVDF, forming a single-phased PVDF–PVP polymer with excellent flowability and uniform microstructure when the PVP content is equal to or higher than 40 wt% due to the effective hydrogen bonding between the functional groups of PVP and PVDF. A proton conductivity of 0.093 S cm−1 was obtained for a 20 wt% PVDF–80 wt% PVP membrane with a H3PO4 doping level of 2.7 at 200 °C under anhydrous conditions, compatible with the state-of-the-art PBI/PA membranes. PEM fuel cells with PA/PVDF–PVP membranes showed a high power density of 530 mW cm−2 at 180 °C in H2/O2 and excellent stability without external humidification. The results indicate the high structural and chemical stability and high retention capability of the blended membranes for doped PA at elevated operation temperatures. The PVDF–PVP hybrids show promising potential as alternative PEMs for fuel cells at elevated high temperatures and under anhydrous conditions.

 Please wait while we load your content...
Please wait while we load your content...