Long-ranged and soft interactions between charged colloidal particles induced by multivalent coions
Abstract
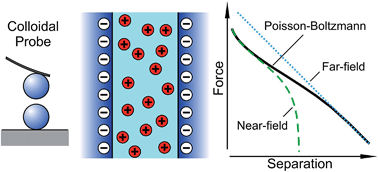
Forces between charged particles in aqueous solutions containing multivalent coions and monovalent counterions are studied by the colloidal probe technique. Here, the multivalent ions have the same charge as the particles, which must be contrasted to the frequently studied case where multivalent ions have the opposite sign as the substrate. In the present case, the forces remain repulsive and are dominated by the interactions of the double layers. The valence of the multivalent coion is found to have a profound influence on the shape of the force curve. While for monovalent coions the force profile is exponential down to separations of a few nanometers, the interaction is much softer and longer-ranged in the presence of multivalent coions. The force profiles in the presence of multivalent coions and in the mixtures of monovalent and multivalent coions can be accurately described by Poisson–Boltzmann theory. These results are accurate for different surfaces and even in the case of highly charged particles. This behavior can be explained by the fact that the force profile follows the near-field limit to much larger distances for multivalent coions than for monovalent ones. This limit corresponds to the conditions with no salt, where the coions are expelled between the two surfaces.

 Please wait while we load your content...
Please wait while we load your content...