Utilizing the electron transfer mechanism of chlorophyll a under light for controlled radical polymerization†
Abstract
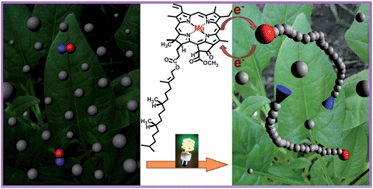
Efficient photoredox catalysts containing transition metals, such as iridium and ruthenium, to initiate organic reactions and polymerization under visible light have recently emerged. However, these catalysts are composed of rare metals, which limit their applications. In this study, we report an efficient photoinduced living radical polymerization process that involves the use of chlorophyll as the photoredox biocatalyst. We demonstrate that chlorophyll a (the most abundant chlorophyll in plants) can activate a photoinduced electron transfer (PET) process that initiates a reversible addition-fragmentation chain transfer (RAFT) polymerization under blue and red LED light (λmax = 461 and 635 nm, respectively). This process controls a wide range of functional and non-functional monomers, and offers excellent control over molecular weights and polydispersities. The end group fidelity was demonstrated by NMR, UV-vis spectroscopy, and successful chain extensions for the preparation of diblock copolymers.

 Please wait while we load your content...
Please wait while we load your content...