Real-time detection of histone deacetylase activity with a small molecule fluorescent and spectrophotometric probe†
Abstract
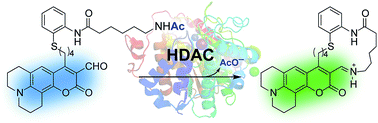
Histone deacetylases (HDACs) are central players in transcription regulation and important targets in cancer treatment. Activity assays are critical tools for the study of the function and regulation of these enzymes, as well as for the screening of potential inhibitors. We report a small-molecule probe for single-step, continuous detection of deacetylase activity based on an acetyl-lysine mimic functionalized with an amine-reactive fluorophore, designed to undergo rapid intramolecular imine formation upon deacetylation. The probe exhibits a bathochromic shift in the absorption spectrum and changes in fluorescence emission intensity that enable unprecedented real-time detection of HDAC activity of purified enzymes or in cell lysates, and offers a means to evaluate HDAC inhibitors via simple spectrophotometric or fluorescence readings without the need of additional reagents.


 Please wait while we load your content...
Please wait while we load your content...