Characterizing chain processes in visible light photoredox catalysis†
Abstract
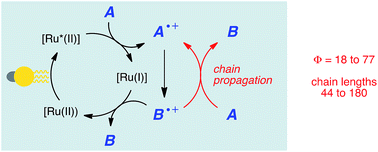
The recognition that Ru(bpy)32+ and similar visible light absorbing transition metal complexes can be photocatalysts for a variety of synthetically useful organic reactions has resulted in a recent resurgence of interest in photoredox catalysis. However, many of the critical mechanistic aspects of this class of reactions remain poorly understood. In particular, the degree to which visible light photoredox reactions involve radical chain processes has been a point of some disagreement that has not been subjected to systematic analysis. We have now performed quantum yield measurements to demonstrate that three representative, mechanistically distinct photoredox processes involve product-forming chain reactions. Moreover, we show that the combination of quantum yield and luminescence quenching experiments provides a rapid method to estimate the length of these chains. Together, these measurements constitute a robust, operationally facile strategy for characterizing chain processes in a wide range of visible light photoredox reactions.
- This article is part of the themed collection: Celebrating 15 years of exceptional research in Chemical Science

 Please wait while we load your content...
Please wait while we load your content...