Catalytic asymmetric direct aldol reaction of α-alkyl azlactones and aliphatic aldehydes†
Abstract
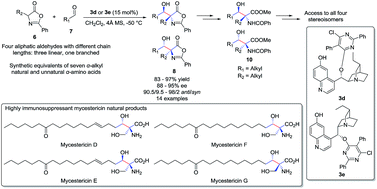
An unprecedented highly diastereoselective and enantioselective aldol reaction of α-alkyl azlactones and aliphatic aldehydes was achieved with cinchona alkaloid catalysts. To our knowledge, this reaction provides the first useful catalytic asymmetric access toward β-hydroxy-α-amino acids bearing alkyl substituents, which are structural motifs embedded in many natural products.


 Please wait while we load your content...
Please wait while we load your content...