Synthesis of sequence-defined acrylate oligomers via photo-induced copper-mediated radical monomer insertions†
Abstract
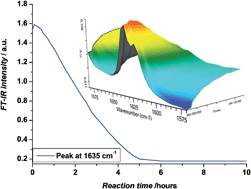
Photo-induced copper-mediated radical polymerization is used to synthesize monodisperse sequence defined acrylate oligomers via consecutive single unit monomer insertion reactions and intermediate purification of the compounds by column chromatography or preparative recycling size exclusion chromatography. Monomer conversions are followed during reaction by means of infrared spectroscopy. When reaction conditions are chosen carefully and any residues from chlorinated solvents are avoided, 100% pure Br end capped sequence defined oligomers are obtained, demonstrating the convenience and power of photo-induced copper mediated radical insertion for establishing sequence control. Within this work, a library of sequence defined oligomers containing polar and apolar ester groups have been obtained, and for the first time, perfectly monodisperse acrylate pentamers became accessible from radical insertion reactions.

 Please wait while we load your content...
Please wait while we load your content...