Designing efficient photochromic dithienylethene dyads†
Abstract
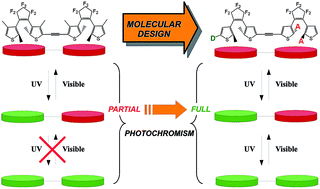
Aiming at designing more efficient multiphotochromes, we investigate with the help of ab initio tools the impact of the substitution on a series of dimers constituted of two dithienylethene (DTE) moieties, strongly coupled to each other through an ethynyl linker. The electronic structure and the optical properties of a large panel of compounds, substituted on different positions by various types of electroactive groups, have been compared with the aim of designing a dyad in which the three possible isomers (open–open, closed–open, closed–closed) can be reached. We show that appending the reactive carbons atoms of the DTE core with electroactive groups on one of the two photochromes allows cyclisation to be induced on a specific moiety, which leads to the formation of the desired closed–open isomer. Substituting the lateral positions of the thiophene rings provides further control of the topology of the frontier molecular orbitals, so that the electronic transition inducing the second ring closure stands out in the spectrum of the intermediate isomer.

 Please wait while we load your content...
Please wait while we load your content...