Combining triazole ligation and enzymatic glycosylation on solid phase simplifies the synthesis of very long glycoprotein analogues†
Abstract
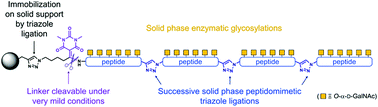
The solid-phase chemical assembly of a protein through iterative chemoselective ligation of unprotected peptide segments can be followed with chemical and/or enzymatic transformations of the resulting immobilized protein, the latter steps thus benefitting from the advantages provided by the solid support. We demonstrate here the usefulness of this strategy for the chemo-enzymatic synthesis of glycoprotein analogues. A linker was specifically designed for application to the synthesis of O-glycoproteins: this new linker is readily cleaved under mild aqueous conditions compatible with very sensitive glycosidic bonds, but is remarkably stable under a wide range of chemical and biochemical conditions. It was utilized for solid-supported N-to-C peptidomimetic triazole ligation followed by enzymatic glycosylation, ultimately leading to a very large MUC1-derived glycoprotein containing 160 amino acid residues, 24 α-GalNAc moieties linked to Ser and Thr, and 3 triazoles as peptide bond mimetics.

 Please wait while we load your content...
Please wait while we load your content...