Molecular glues for manipulating enzymes: trypsin inhibition by benzamidine-conjugated molecular glues†
Abstract
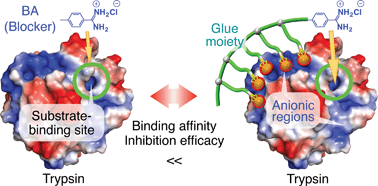
Water-soluble bioadhesive polymers bearing multiple guanidinium ion (Gu+) pendants at their side-chain termini (Gluen–BA, n = 10 and 29) that were conjugated with benzamidine (BA) as a trypsin inhibitor were developed. The Gluen–BA molecules are supposed to adhere to oxyanionic regions of the trypsin surface, even in buffer, via a multivalent Gu+/oxyanion salt-bridge interaction, such that their BA group properly blocks the substrate-binding site. In fact, Glue10–BA and Glue29–BA exhibited 35- and 200-fold higher affinities for trypsin, respectively, than a BA derivative without the glue moiety (TEG–BA). Most importantly, Glue10–BA inhibited the protease activity of trypsin 13-fold more than TEG–BA. In sharp contrast, mGlue27–BA, which bears 27 Gu+ units along the main chain and has a 5-fold higher affinity than TEG–BA for trypsin, was inferior even to TEG–BA for trypsin inhibition.

 Please wait while we load your content...
Please wait while we load your content...