Oxalyl amide assisted palladium-catalyzed synthesis of pyrrolidones via carbonylation of γ-C(sp3)–H bonds of aliphatic amine substrates†
Abstract
The first Pd-catalyzed regioselective γ-carbonylation of oxalyl amide protected aliphatic amines with carbon monoxide leading to synthesis of pyrrolidones has been developed. Both γ-methyl and cyclopropyl methylene C–H bonds are well activated to obtain the corresponding pyrrolidones in moderate to excellent yields. The role of 3-(trifluoromethyl)benzoic acid as an additive is critical as it helps in stabilizing the palladium intermediate formed during the catalytic cycle. The reaction scope is extended to benzylamine and allyl amine derivatives, thereby affording the corresponding products in good to excellent yields.
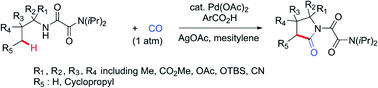
- This article is part of the themed collection: SU 120: Celebrating 120 Years of Soochow University

 Please wait while we load your content...
Please wait while we load your content...