Unexpected effect of catalyst concentration on photochemical CO2 reduction by trans(Cl)–Ru(bpy)(CO)2Cl2: new mechanistic insight into the CO/HCOO− selectivity†
Abstract
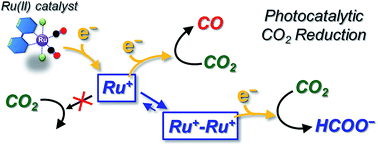
Photochemical CO2 reduction catalysed by trans(Cl)–Ru(bpy)(CO)2Cl2 (bpy = 2,2′-bipyridine) efficiently produces carbon monoxide (CO) and formate (HCOO−) in N,N-dimethylacetamide (DMA)/water containing [Ru(bpy)3]2+ as a photosensitizer and 1-benzyl-1,4-dihydronicotinamide (BNAH) as an electron donor. We have unexpectedly found catalyst concentration dependence of the product ratio (CO/HCOO−) in the photochemical CO2 reduction: the ratio of CO/HCOO− decreases with increasing catalyst concentration. The result has led us to propose a new mechanism in which HCOO− is selectively produced by the formation of a Ru(I)–Ru(I) dimer as the catalyst intermediate. This reaction mechanism predicts that the Ru–Ru bond dissociates in the reaction of the dimer with CO2, and that the insufficient electron supply to the catalyst results in the dominant formation of HCOO−. The proposed mechanism is supported by the result that the time-course profiles of CO and HCOO− in the photochemical CO2 reduction catalysed by [Ru(bpy)(CO)2Cl]2 (0.05 mM) are very similar to those of the reduction catalysed by trans(Cl)–Ru(bpy)(CO)2Cl2 (0.10 mM), and that HCOO− formation becomes dominant under low-intensity light. The kinetic analyses based on the proposed mechanism could excellently reproduce the unusual catalyst concentration effect on the product ratio. The catalyst concentration effect observed in the photochemical CO2 reduction using [Ru(4dmbpy)3]2+ (4dmbpy = 4,4′-dimethyl-2,2′-bipyridine) instead of [Ru(bpy)3]2+ as the photosensitizer is also explained with the kinetic analyses, reflecting the smaller quenching rate constant of excited [Ru(4dmbpy)3]2+ by BNAH than that of excited [Ru(bpy)3]2+. We have further synthesized trans(Cl)–Ru(6Mes-bpy)(CO)2Cl2 (6Mes-bpy = 6,6′-dimesityl-2,2′-bipyridine), which bears bulky substituents at the 6,6′-positions in the 2,2′-bipyridyl ligand, so that the ruthenium complex cannot form the dimer due to the steric hindrance. We have found that this ruthenium complex selectively produces CO, which strongly supports the catalytic mechanism proposed in this work.

 Please wait while we load your content...
Please wait while we load your content...