Asymmetric Lewis acid catalysis directed by octahedral rhodium centrochirality†
Abstract
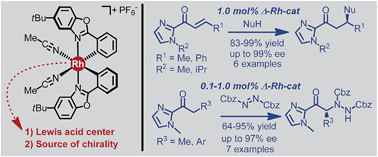
A rhodium-based asymmetric catalyst is introduced which derives its optical activity from octahedral centrochirality. Besides providing the exclusive source of chirality, the rhodium center serves as a Lewis acid by activating 2-acyl imidazoles through two point binding and enabling a very effective asymmetric induction mediated by the propeller-like C2-symmetrical ligand sphere. Applications to asymmetric Michael additions (electrophile activation) as well as asymmetric α-aminations (nucleophile activation) are disclosed, for which the rhodium catalyst is found to be overall superior to its iridium congener. Due to its straightforward proline-mediated synthesis, high catalytic activity (catalyst loadings down to 0.1 mol%), and tolerance towards moisture and air, this novel class of chiral-at-rhodium catalysts will likely to become of widespread use as chiral Lewis acid catalysts for a large variety of asymmetric transformations.

 Please wait while we load your content...
Please wait while we load your content...