Annihilation electrogenerated chemiluminescence of mixed metal chelates in solution: modulating emission colour by manipulating the energetics
Abstract
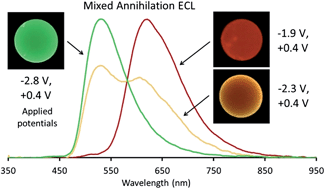
We demonstrate the mixed annihilation electrogenerated chemiluminescence of tris(2,2′-bipyridine)ruthenium(II) with various cyclometalated iridium(III) chelates. Compared to mixed ECL systems comprising organic luminophores, the absence of T-route pathways enables effective predictions of the observed ECL based on simple estimations of the exergonicity of the reactions leading to excited state production. Moreover, the multiple, closely spaced reductions and oxidations of the metal chelates provide the ability to finely tune the energetics and therefore the observed emission colour. Distinct emissions from multiple luminophores in the same solution are observed in numerous systems. The relative intensity of these emissions and the overall emission colour are dependent on the particular oxidized and reduced species selected by the applied electrochemical potentials. Finally, these studies offer insights into the importance of electronic factors in the question of whether the reduced or oxidized partner becomes excited in annihilation ECL.

 Please wait while we load your content...
Please wait while we load your content...