Hydrogen dangling bonds induce ferromagnetism in two-dimensional metal-free graphitic-C3N4 nanosheets†
Abstract
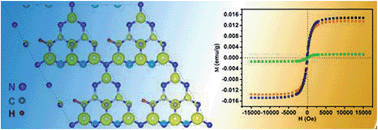
Ferromagnetic two-dimensional (2D) ultrathin nanosheets hold great promise for next generation electronics. Ferromagnetic metal-free materials that usually possess only an s/p electronic configuration with weak spin–orbit coupling and a large spin relaxation time, would play an important role in constructing future spintronic devices. However, the absence of an intrinsic spin ordering structure in most metal-free materials greatly hampers the widening scope of ferromagnetic 2D nanostructures as well as in-depth understanding of their ferromagnetic nature. Herein, the induction of intrinsic ferromagnetism in 2D metal-free g-C3N4 ultrathin nanosheets has been achieved through a new effective strategy whereby hydrogen dangling bonds are introduced. In our case, g-C3N4 ultrathin nanosheets with hydrogen dangling bonds showed obvious room temperature ferromagnetic behavior that could even be tuned by the concentration of hydrogen. This work will pave a new pathway to engineer the properties of 2D nanomaterial systems.

 Please wait while we load your content...
Please wait while we load your content...