Accelerated Hantzsch electrospray synthesis with temporal control of reaction intermediates†
Abstract
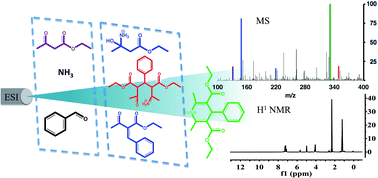
Complex chemical reactions can occur in electrosprayed droplets on the millisecond time scale. The Hantzsch synthesis of 1,4-dihydropyridines was studied in this way using on-line mass spectral analysis to optimize conditions and characterize the product mixture. Changing the distance between the nanospray source and the MS inlet allowed exploration of reaction progress as a function of droplet time-of-flight. Desolvation of the charged microdroplets is associated with transformation from starting material to intermediates and eventually to product as the distance is increased. Results of the on-line experiments require a termination step that discontinuously completes the desolvation process and allows the generated gaseous ions to be used to characterize the state of the system at a particular time. The intermediates seen correspond to those known to occur in the bulk solution-phase reaction. Off-line collection of the sprayed reaction mixture allowed the recovery of 250 mg h−1 of desired reaction product from a single sprayer, permitting characterization by NMR and other standard methods. A thin film version of the accelerated reaction is described and it could be controlled through the temperature of the collection surface.

 Please wait while we load your content...
Please wait while we load your content...