Structural study of a small molecule receptor bound to dimethyllysine in lysozyme†
Abstract
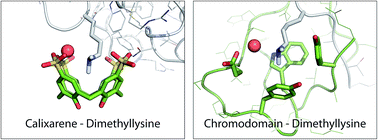
Lysine is a ubiquitous residue on protein surfaces. Post translational modifications of lysine, including methylation to the mono-, di- or trimethylated amine result in chemical and structural alterations that have major consequences for protein interactions and signalling pathways. Small molecules that bind to methylated lysines are potential tools to modify such pathways. To make progress in this direction, detailed structural data of ligands in complex with methylated lysine is required. Here, we report a crystal structure of p-sulfonatocalix[4]arene (sclx4) bound to methylated lysozyme in which the lysine residues were chemically modified from Lys-NH3+ to Lys-NH(Me2)+. Of the six possible dimethyllysine sites, sclx4 selected Lys116-Me2 and the dimethylamino substituent was deeply buried in the calixarene cavity. This complex confirms the tendency for Lys-Me2 residues to form cation–π interactions, which have been shown to be important in protein recognition of histone tails bearing methylated lysines. Supporting data from NMR spectroscopy and MD simulations confirm the selectivity for Lys116-Me2 in solution. The structure presented here may serve as a stepping stone to the development of new biochemical reagents that target methylated lysines.

 Please wait while we load your content...
Please wait while we load your content...