Stoichiometric to catalytic reactivity of the aryl cycloaurated species with arylboronic acids: insight into the mechanism of gold-catalyzed oxidative C(sp2)–H arylation†
Abstract
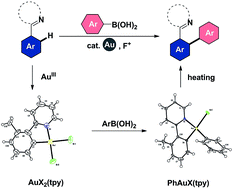
Based on the well-defined five-membered aryl gold(III) complexes, [Au(tpy)X2] (3a and 3b) and [AuBr(Ph)(tpy)] (7), as well as the aryl gold(III) complex [AuCl2(Ph)(tpy)] (8) (tpy = 2-(o-tolyl)pyridine) as reliable models, we present a detailed study of the mechanism for gold(III)-catalyzed oxidative cross-coupling reactions between cycloaurable arenes and arylboronic acids. Here we report the direct evidence for a mechanistic proposal including arene C–H activation, transmetallation and biaryl reductive elimination. The chelation-assisted C–H activation strategy has been used for the development of the gold(III)-catalyzed C–H bond arylation of arenes with aryl reagents to forge extended π-conjugated systems.

 Please wait while we load your content...
Please wait while we load your content...