Evaluation of the anticancer properties of the predicted hBaxBH3-mimetic compound 2-hydroxy-3,5-dinitrobenzamide in a mammary carcinogenesis-induced rat model†
Abstract
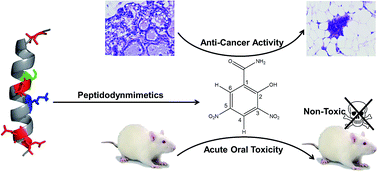
Designing small molecular prototypes having potential to disrupt binding interfaces of pro-apoptotic–anti-apoptotic/BH3-only proteins is a promising strategy in cancer chemotherapy. Several natural and synthetic chemical compounds have been reported as inhibitors or BH3-mimetics to anti-apoptotic proteins of the Bcl-2 family playing essential roles in apoptosis. In the present study, chemical synthesis, structural characterizations and anti-cancer effect of 2-hydroxy-3,5-dinitrobenzamide (HDNB), a chemical compound identified on the basis of pharmacophoric features of hBaxBH3 peptide have been systematically reported. The chemopreventive effect of the HDNB was assessed on N-nitroso-N-methylurea-induced Wistar female rats of mammary gland carcinogenesis for the study period of 18 weeks. The carcinogenesis Wistar rats were subjected to oral administration of a low-dose (8 mg kg−1 b.w.), medium-dose (40 mg kg−1 b.w.) and high-dose (200 mg kg−1 b.w.) of the HDNB in three different experimental setups. Strikingly, a medium-dose (40 mg kg−1 b.w.) of the HDNB showed appreciable potential to reverse the abnormalities of various biochemical parameters in blood samples and as well in breast tissues to the values close to that of healthy control or to that of the standard (tamoxifen) control. The chemopreventive potential of the HDNB could be chiefly attributed to its ability to restore antioxidants and as well apoptosis inducing properties in Wistar animal models.

 Please wait while we load your content...
Please wait while we load your content...