Investigation of luminescence properties and the energy transfer mechanism of Li6Lu(BO3)3:Ce3+, Tb3+ green-emitting phosphors
Abstract
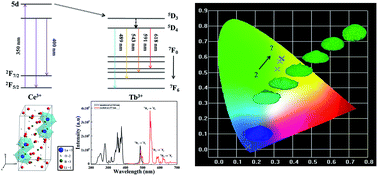
Li6Lu(BO3)3:Ce3+, Tb3+ phosphor was synthesized via a conventional solid-state method. The phase purity, photoluminescence properties, energy transfer mechanism, thermal stability and chromaticity coordinates were investigated. The absorption spectrum was comprised of broad bands in the UV region and the emission spectrum was consisted of characteristic peaks from both Ce3+ and Tb3+ under UV excitation. The amount of Ce3+ was fixed to 3 mol% while Tb3+ was varied from 20 to 80 mol% and the chromaticity coordinates were tuned from the blue to green region. The energy transfer mechanism between Ce3+ and Tb3+ was attributed to a quadrupole–quadrupole interaction and the critical distance was measured to be 8.12 Å. The overall performance of the phosphor was enhanced by the efficient energy transfer between Ce3+ and Tb3+ ions.

 Please wait while we load your content...
Please wait while we load your content...