Pd-functionalized MCM-41 nanoporous silica as an efficient and reusable catalyst for promoting organic reactions†
Abstract
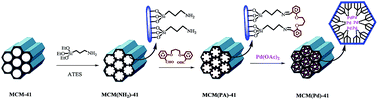
Pd-functionalized MCM-41 nanoporous silica has been explored as an efficient and recyclable catalyst to effect Suzuki and Mizoroki–Heck cross-coupling reactions, under various conditions. In Suzuki–Miyaura reactions, various aryl halides were coupled with aryl boronic acids at 100 °C under solvent-free conditions using K2CO3 as base and the maximum Ar–Ar yield reached 95%. The MCM(Pd)-41 catalyst was also used for a good range of Heck reactions using different aryl halides with styrene or n-butyl acrylate esters at 130 °C under solvent-free conditions using n-Pr3N as base that gave the maximum yields of 95% and 92%, respectively. High yield, low reaction times, non-toxicity and recyclability of the catalyst are the main merits of this protocol. The catalyst has been characterized by FT-IR, XRD, SEM, TEM, XPS, EDX, ICP-AES, TG and N2 adsorption–desorption.

 Please wait while we load your content...
Please wait while we load your content...