Green coating by coordination of tannic acid and iron ions for antioxidant nanofiltration membranes†
Abstract
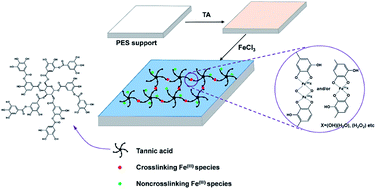
A novel green coating method was proposed to prepare composite nanofiltration (NF) membranes without using organic solutions or toxic reagents in the formation of the active layer compared with traditional interfacial polymerization. Tannic acid (TA) and iron(III) chloride (FeCl3) were chosen as the two reactive monomers dissolved in the aqueous phase. The stable metal–polyphenol complex coating was formed via the coordination reaction between TA and iron ions (FeIII) upon porous support. Fourier transform infrared (FTIR) spectroscopy, X-ray photoelectron spectroscopy (XPS), and water contact angle were used to characterize the chemical features of the prepared TA–FeIII/polyethersulfone (PES) composite NF membranes. Scanning electron microscope (SEM) and atomic force microscopy (AFM) were utilized to observe the surface morphologies. The effects of reactive monomer concentration and reaction time on the permeability of water and rejection of dyes and inorganic salts were investigated, respectively. The TA–FeIII/PES composite NF membranes possessed good structural stability and oxidation resistance ability.

 Please wait while we load your content...
Please wait while we load your content...