Immobilizing and de-immobilizing enzymes on mesoporous silica†
Abstract
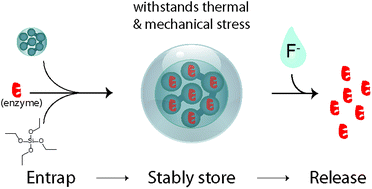
Beta glucosidase was immobilised as a model enzyme within mesoporous silica (MCF) at a high loading (80 mg g−1). The enzyme was further entrapped within the material by precipitating additional silica within the channels. This entrapment was performed by the polycondensation of tetraethoxysilane under very mild conditions (pure water). Although unreactive while entrapped, in this state the enzyme was highly stable towards heat treatments of 60–70 °C. Upon release from the matrix by a mild silica dissolution step involving a fluoride comprising buffer, the enzyme regained most of its original activity. With this we developed a novel protein entrapment/release scheme, which is designed along the principles of orthogonal protection group chemistry as the protection/deprotection steps do not affect the integrity of the (bio)molecule. The principle can be adopted to many previously developed mesoporous silica/enzyme biocomposites and will allow the application of enzyme dependent diagnostic devices in applications involving demanding environmental storage requirements.


 Please wait while we load your content...
Please wait while we load your content...