Cinchona alkaloid thiourea mediated asymmetric Mannich reaction of isocyanoacetates with isatin-derived ketimines and subsequent cyclization: enantioselective synthesis of spirooxindole imidazolines†
Abstract
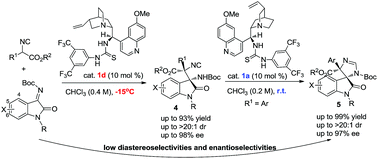
We report the organocatalyzed asymmetric Mannich reaction of isocyanoacetates with isatin derived ketimines in good yields along with high stereoselectivities. The subsequent organocatalyzed cyclization of Mannich adducts is also investigated, emerging as a promising strategy for the synthesis of optically active spirooxindole imidazolines.

 Please wait while we load your content...
Please wait while we load your content...