Comparative analysis of the electronic structures of mono- and bi-atomic chains of IV, III–V and II–VI group elements calculated using the DFT LCAO and LACW methods
Abstract
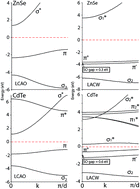
Using the first principle non-relativistic linear combination of atomic orbitals (LCAO) and relativistic linearized augmented cylindrical wave (LACW) methods, the band structure of the covalent and partially ionic ANB8−N single atom width chain is calculated. Both the LCAO and LACW methods show that the chains of C, Si, Ge, Sn, and Pb are metallic. However, there is a great difference between the relativistic and non-relativistic band structures. The π bands crossing the Fermi level are orbitally doubly degenerate in the non-relativistic model. The relativistic LACW calculations demonstrate that the spin and orbital motion of electrons are coupled, thereby splitting the π bands. The spin–orbit gaps are equal to 1.5 meV, 28 meV, 0.22 eV, 0.45 eV, and 4 eV for the C, Si, Ge, Sn, and Pb chains, respectively. The mass–velocity corrections result in a lowering of all the valence band levels. In the carbon and silicon chains, the corrections are possibly negligible (2–5 and 10–30 meV, respectively), while in the Ge, Sn, and Pb chains the low-energy shifts are equal to 0.6, 2.2, and 3.7 eV, respectively, due to these effects. The Darwin corrections are several times smaller in comparison to the mass–velocity contributions. The transition from the covalent chains to the partially ionic ones is accompanied by a drastic change in the band structure. The C chain with all bond lengths equal has a metal type electronic structure while the BN chain is an insulator with an energy gap equal to 6–8 eV. The differences between the covalent and partially ionic chains are explained by the presence of the antisymmetric components of the electron potential in the latter case. The transition from the BN chain to the AlP, GaAs, and InSb ones is accompanied by a gradual decrease in the gaps; for example, the AlP chain is a semiconductor. According to the LCAO calculations, the GaAs chain is a semiconductor, but it is a metal according to the relativistic LACW method. The InSb chain possesses a metal type band structure, but the spin–orbit interaction splits the π states, forming the two π+ and π− sub-bands, and noticeably complicates the band structure and density of states in the vicinity of the Fermi level. In the case of compounds from the same horizontal row in the periodic table, the transition from the AIIIBV chains to the AIIBVI ones is accompanied by a sharp increase in the band gap. The calculations indicate the metallic nature of the InSe chain, but the CdTe one is an insulator. Among the atomic ANB8−N chains, there are compounds with different electrical properties: from metals to semiconductors and insulators.

 Please wait while we load your content...
Please wait while we load your content...