Cross-strand disulfides in the non-hydrogen bonding site of antiparallel β-sheet (aCSDns): poised for biological switching†
Abstract
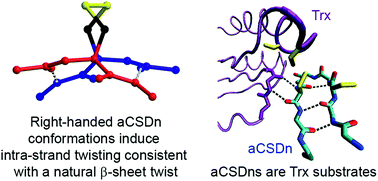
Forbidden disulfides are stressed disulfides found in recognisable protein contexts previously defined as structurally forbidden. The torsional strain of forbidden disulfides is typically higher than for structural disulfides, but not so high as to render them immediately susceptible to reduction under physionormal conditions. The meta-stability of forbidden disulfides makes them likely candidates as redox switches. Here we mined the Protein Data Bank for examples of the most common forbidden disulfide, the aCSDn. This is a canonical motif in which disulfide-bonded cysteine residues are positioned directly opposite each other on adjacent anti-parallel β-strands such that the backbone hydrogen bonded moieties are directed away from each other. We grouped these aCSDns into homologous clusters and performed an extensive physicochemical and informatic analysis of the examples found. We estimated their torsional energies using quantum chemical calculations and studied differences between the preferred conformations of the computational model and disulfides found in solved protein structures to understand the interaction between the forces imposed by the disulfide linkage and typical constraints of the surrounding β-sheet. In particular, we assessed the twisting, shearing and buckling of aCSDn-containing β-sheets, as well as the structural and energetic relaxation when hydrogen bonds in the motif are broken. We show the strong preference of aCSDns for the right-handed staple conformation likely arises from its compatibility with the twist, shear and Cα separation of canonical β-sheet. The disulfide can be accommodated with minimal distortion of the sheet, with almost all the strain present as torsional strain within the disulfide itself. For each aCSDn cluster, we summarise the structural and strain data, taxonomic conservation and any evidence of redox activity. aCSDns are known substrates of thioredoxin-like enzymes. This, together with their meta-stability, means they are ideally suited to biological switching roles and are likely to play important roles in the molecular pathways of oxidative stress.

 Please wait while we load your content...
Please wait while we load your content...