Study on the aqueous solution behavior and extraction mechanism of Nd(iii) in the presence of the complexing agent lactic acid with di-(2-ethylhexyl) phosphoric acid
Abstract
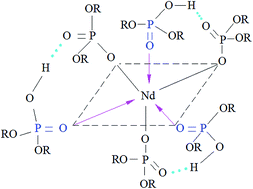
The aqueous solution behavior and extraction mechanism of single Nd(III) from a chloride medium with di-(2-ethylhexyl) phosphoric acid (D2EHPA, H2A2) in the presence of the complexing agent lactic acid (HLac) have been reported. The analyses by FT-IR and UV of the aqueous solution indicate that there is a coordination mechanism between Nd3+ and Lac− in the form of the carboxyl oxygen bridge bidentate ligand. A cation extraction mechanism has been studied using the method of slope analysis and saturation loading capacities, and also confirmed by FT-IR and NMR. The equilibrium constants and thermodynamic functions have been calculated. The separation factors of La/Ce, Ce/Pr and Pr/Nd are 3.24, 2.04 and 1.58, indicating that this system is beneficial for separating light rare earths. The results could provide some reference value for complexing extraction systems.

 Please wait while we load your content...
Please wait while we load your content...