Redox-sensitive mesoporous silica nanoparticles functionalized with PEG through a disulfide bond linker for potential anticancer drug delivery
Abstract
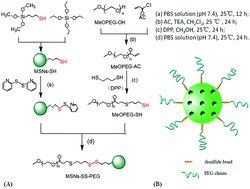
In this paper, redox-sensitive mesoporous silica nanoparticles functionalized with polyethylene glycol (PEG) through a disulfide bond linker (MSNs–SS–PEG) were successfully synthesized using silica nanoparticles modified with a thiol group (MSNs–SH) and thiol-functionalized methoxy polyethylene glycol (MeOPEG–SH). Meanwhile, the particle size, pore size and structural properties of these materials were characterized by transmission electron microscopy (TEM), field emission scanning electron microscopy (FE-SEM), dynamic light scattering (DLS), Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD) and nitrogen adsorption–desorption measurements. Furthermore, the in vitro drug release behaviour of DOX-loaded MSNs–SS–PEG (DOX@MSNs–SS–PEG) was investigated. It was shown that DOX release was markedly accelerated with the increasing concentration of glutathione (GSH), while DOX was not released from the carrier materials in the absence of GSH. Cytotoxicity evaluation revealed the good biocompatibility of the blank nanoparticles and the DOX@MSNs–SS–PEG exhibited comparative anticancer activity to free DOX towards BEL-7402 cells. Therefore, the MSNs–SS–PEG might be a great potential carrier for anticancer drug delivery.

 Please wait while we load your content...
Please wait while we load your content...