Multifunctional poly(phosphoester)s with two orthogonal protective groups†
Abstract
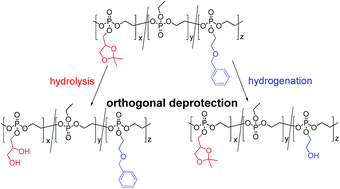
A novel cyclic phosphate monomer, 2-(2-(benzyloxy)ethoxy)-1,3,2-dioxaphospholane-2-oxide (BnEEP), was developed to generate poly(phosphoester)s containing protected pendant hydroxyl groups by anionic ring-opening polymerization. The hydroxyl-groups were released by a mild catalytic hydrogenation leaving the polymer backbone intact. In addition, the number of pendant hydroxyl groups was varied by copolymerization of BnEEP with ethyl ethylene phosphate (EEP). Furthermore, copolymers of BnEEP with an acetal protected cyclic phosphate, 2-(2,2-dimethyl-1,3-dioxolan-4-yl-methoxy)-2-oxo-1,3,2-dioxaphospholane (GEP), were prepared in order to establish a selective deprotection of the acetal or the benzyl protective groups by acidic hydrolysis or catalytic hydrogenation respectively. No degradation of the polyester backbone was detected under the reported conditions. The novel monomer allows adjustment of the chemical and physical properties of the poly(phosphoester)s and gives access to various side chain functionalities.

 Please wait while we load your content...
Please wait while we load your content...