Nanotoxicity of polyelectrolyte-functionalized titania nanoparticles towards microalgae and yeast: role of the particle concentration, size and surface charge†
Abstract
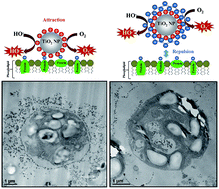
We studied the nanotoxicity of titania nanoparticles (TiO2NPs) of various hydrodynamic diameters and crystallite sizes towards C. reinhardtii microalgae and S. cerevisiae (yeast) upon illumination with UV and visible light. The cell viability was assessed for a range of nanoparticle concentrations and incubation times. We found that bare TiO2NPs affect the C. reinhardtii cell viability at much lower particle concentrations than for yeast. We observed an increase of the TiO2NPs toxicity upon illumination with UV light compared with that in dark conditions due to the oxidative stress of the produced reactive oxygen species. We also found an increased TiO2NPs nanotoxicity upon illumination with visible light which indicates that they may also interfere with the microalgae's photosynthetic system leading to decreased chlorophyll content upon exposure to TiO2NPs. The results indicate that the larger the hydrodynamic diameter of the TiO2NPs the lower is their nanotoxicity, with anatase TiO2NPs generally being more toxic than rutile TiO2NPs. We also prepared a range of polyelectrolyte-coated TiO2NPs using a layer by-layer method and studied their nanotoxicity towards yeast and microalgae. We found that the toxicity of the coated TiO2NPs changes with their surface charge. TiO2NPs coated with cationic polyelectrolyte as an outer layer exhibit much higher nanotoxicity than the ones with an outer layer of anionic polyelectrolyte. TEM images of sectioned microalgae and yeast cells exposed to different polyelectrolyte-coated TiO2NPs confirmed the formation of a significant build-up of nanoparticles on the cell surface for bare and cationic polyelectrolyte-coated TiO2NPs. The effect comes from the increased adhesion of cationic nanoparticles to the cell walls. Significantly, coating the TiO2NPs with anionic polyelectrolyte as an outer layer led to a reduced adhesion and much lower nanotoxicity due to electrostatic repulsion with the cell walls. This suggest a new way of making cationic TiO2NPs safer for use in different formulations by pre-coating them with anionic polyelectrolytes. The results of this study give important insights into the various factors controlling the nanotoxicity of TiO2NPs.

 Please wait while we load your content...
Please wait while we load your content...