Reaction routes in catalytic reforming of poly(3-hydroxybutyrate) into renewable hydrocarbon oil†
Abstract
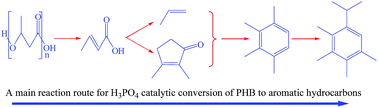
Poly(3-hydroxybutyrate) or PHB is an energy storage material of microbial organisms and can be reformed into hydrocarbon oils rich with aromatic compounds. This work investigated the main reaction routes from PHB to the key intermediates and final hydrocarbons. The main sequential reactions under catalysis of phosphoric acid at moderate temperatures (200–230 °C) consist of: (1) decomposition of PHB into crotonic acid, a major monomeric intermediate, (2) deoxygenation of crotonic acid, and (3) combination of the deoxygenated molecules. The oxygen in PHB is removed as CO2 and H2O in stage (2), involving decarboxylation and ketonization of crotonic acid. The main aromatic compounds are formed in stage (3) from propylene and 2,3-dimethyl-2-cyclopenten-1-one as two key intermediates, the former from decarboxylation and the latter from ketonization of crotonic acid. The reaction routes reveal that the formation of aromatics is affected to a great extent by the concentrations of phosphoric acid and water in the reaction, which can be used to control the composition of hydrocarbon oil.

 Please wait while we load your content...
Please wait while we load your content...