Synthesis of electrocrystallized cobalt ferrite nanopowders by tuning the cobalt salt concentration
Abstract
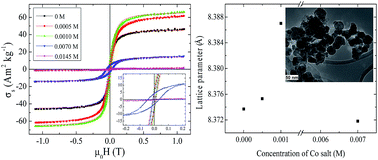
Cobalt ferrite nanopowders were synthesized by an electrocrystallization method. The synthesis was carried out in an electrochemical cell containing two iron electrodes, and an electrolyte solution of sodium sulfate, sodium butanoate, and cobalt sulphate heptahydrate. The concentration of the cobalt salt was optimized to produce cobalt ferrite nanoparticles. The samples were characterized by X-ray diffraction, XPS analysis, electron microscopy, magnetometry and Mössbauer spectroscopy. Based on XRD and XPS results we found that the cobalt ferrite nanoparticles are formed in the electrochemical cell containing 0.007 M cobalt sulphate heptahydrate. The nanoparticle size, shape, and morphology were characterized using electron microscopy. Electron microscope images of the samples show the mean particle size ranges from ∼7 nm to ∼90 nm. Magnetization curves of the samples show that the coercivity of the cobalt ferrite sample is about 0.04 T, which is much higher than the other samples. Based on our Mössbauer analysis it was found that increasing the concentration of cobalt, dramatically affects the subspectra. An increase in the hyperfine field and decrease in the population of octahedral sites provide direct evidence for the presence of the cobalt ions substitution in the octahedral sites.

 Please wait while we load your content...
Please wait while we load your content...