Electrochemistry for the generation of renewable chemicals: electrochemical conversion of levulinic acid†
Abstract
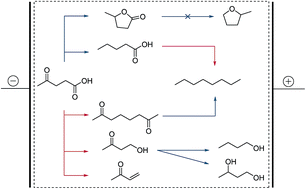
The oxidative and reductive electrochemical conversion of levulinic acid to its primary products valeric acid, γ-valerolactone, 2,7-octanedione, 4-hydroxy-2-butanone and 3-buten-2-one is studied in detail. The reactions were performed in aqueous solutions and at ambient temperature, following the principles of green chemistry. The obtained primary reaction products were studied with respect to the oxidative and reductive electrochemical formation of secondary products, such as n-octane, 1-butanol and 1,3-butanediol. It is shown that the choice of electrolyte composition, educt concentration and the nature of the electrode material has a strong influence on the selectivity of product formation. For instance it is demonstrated that in alkaline solutions γ-valerolactone can be gained from levulinic acid at iron electrodes with similar Coulombic efficiency (∼20%) but higher selectivity (S = 70%) than on lead (S = 50%). Furthermore, for the first time the electrochemical two-step reaction of levulinic acid to 1-butanol via 4-hydroxy-2-butanone is reported. For some of the reaction pathways the main product is water insoluble, which allows a direct separation of the product and the potential electrolyte reuse in a semi-continuous process. Especially the use of the electrocatalytic hydrogenation may provide a path for the storage of electricity into liquid organic fuels as shown by a basic energetic assessment of all electrochemical conversions.

 Please wait while we load your content...
Please wait while we load your content...