Removal of Malachite Green from water using hydrothermally carbonized pine needles†
Abstract
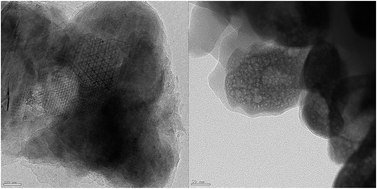
Hydrothermal carbonization of pine needles (HTC-PN) and their oxidized-activated form HTC-APN are prepared and applied for the adsorption of Malachite Green (MG) in aqueous solution. The HTC materials were characterized by thermal and TEM analysis. Adsorbent dose, initial concentration of MG, contact time, temperature and pH effect on MG adsorption onto the HTC materials were studied. The adsorption equilibrium data was best fitted by the Langmuir isotherm model and the adsorption kinetics followed pseudo-second-order models for both HTC-PN and HTC-APN. The maximum capacity predicted by the Langmuir nonlinear model is 52.91 and 97.08 mg g−1 for uptake of MG by HTC-PN and HTC-APN, respectively, at 30 °C. Thermodynamic investigations showed that the adsorption is spontaneous and endothermic in nature. Results suggest HTC-APN can be used as a low-cost adsorbent for MG removal from industrial wastewater. Yoon–Nelson is the best model with a column capacity of 38.3 mg g−1 for the adsorption of MG onto HTC-APN.

 Please wait while we load your content...
Please wait while we load your content...