Mechanism of a green graphene oxide reduction with reusable potassium carbonate†
Abstract
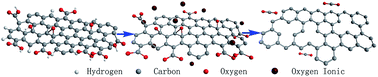
A green method for the deoxygenation of graphene oxide (GO) was developed using K2CO3 as a reusable reduction agent. The size and thickness of the reduced GO are less than 1 μm and around 0.85 nm, respectively. Carbon dioxide is the only byproduct during this process. The reduction mechanism of the graphene oxide includes two reduction steps. On the one hand, ionic oxygen generated from the electrochemical reaction between hydroxyl ions and oxygen in the presence of K2CO3 reacts with carbonyl groups attached to the GO layers at 50 °C. On the other hand, ionic oxygen attacks hydroxyl and epoxide groups, which become carbonyl groups and then are converted to carbon dioxide by K2CO3 at 90 °C. These oxygenous groups are finally converted to CO2 from graphene layers, leading to the formation of graphene sheets. Headspace solid-phase microextraction and gas chromatography-mass spectrometry detected the existence of n-dodecanal and 4-ethylbenzoic acid cyclopentyl ester during the reduction, suggesting that oxygen functional groups on the GO layers are not only aligned, but randomly dispersed in some areas based on the proposed mechanism.

 Please wait while we load your content...
Please wait while we load your content...