An environmentally friendly approach to the green synthesis of azo dyes in the presence of magnetic solid acid catalysts
Abstract
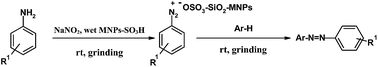
A solvent-free, efficient and green approach for the synthesis of azo dyes has been developed by the diazo coupling reactions of aromatic amines with β-naphthol in the presence of sulfonic acid functionalized magnetic Fe3O4 nanoparticles (Fe3O4@SiO2-SO3H) by a grinding method at room temperature. This green methodology aims to overcome the limitations and drawbacks of the previously reported methods such as low temperature, use of acids, alkalis and toxic solvents, instability of diazonium salts at room temperature, modest yields, and long reaction times. Moreover, the attractive advantages of the process include mild conditions with excellent conversions, simple product isolation process, inexpensive procedure and recyclability of the magnetic catalyst.

 Please wait while we load your content...
Please wait while we load your content...