Synthesis, stability and electrical properties of new soluble pentacenes with unsaturated side groups
Abstract
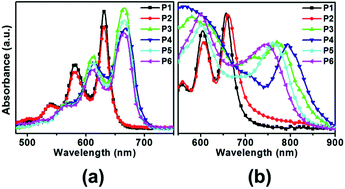
A series of pentacene derivatives with unsaturated hydrocarbon substituents at the 6,13-positions as side groups have been synthesized. The effects of conjugated π-bonding throughout the side groups on the solubility, oxidative stability, crystallinity, and electrical properties of the compounds were investigated. Here we report that the solubility of pentacene can be greatly improved by the insertion of linear long side groups at the 6,13-positions: the obtained pentacene derivatives were soluble in common organic solvents. Time-dependent UV-visible spectra in chloroform solutions as well as thin films were recorded to investigate the oxidative stability of each pentacene compound under ambient conditions. The photooxidative stability of the pentacene derivatives was remarkably enhanced by increasing the electron conjugation length through the installation of unsaturated hydrocarbon substituents at the 6,13-positions. The solution-processed TFT devices based on 6,13-bis(4-pentylphenyl-ethynyl)pentacene (P5) and 6,13-bis(4-hexylphenylethynyl)pentacene (P6) showed charge transport mobilities of 2.14 × 10−2 cm2 V−1 s−1 and 3.94 × 10−2 cm2 V−1 s−1, respectively, and exhibited an on/off ratio of 105. Here we demonstrate that P5 and P6 revealed much more stable electrical performances than the parent pentacene under the oxidative environment.

 Please wait while we load your content...
Please wait while we load your content...