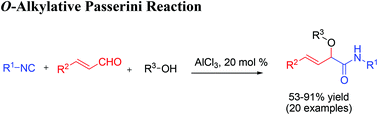
AlCl3-catalyzed O-alkylative Passerini reaction of isocyanides, cinnamaldehydes and various aliphatic alcohols for accessing α-alkoxy-β,γ-enamides†
Abstract
The inexpensive Lewis acid AlCl3 was found to be an efficient catalyst for the O-alkylative Passerini reaction of isocyanides, cinnamaldehydes and alcohols. Instead of carboxylic acid in the classical Passerini reaction, alcohols performed both as the solvent and substrate nicely to afford α-alkoxy-amide products in good yield (up to 91%). This method provides practical access for functional α-alkoxy-β,γ-enamide derivatives.

 Please wait while we load your content...
Please wait while we load your content...