Remendable thermosetting polymers for isocyanate-free adhesives: a preliminary study
Abstract
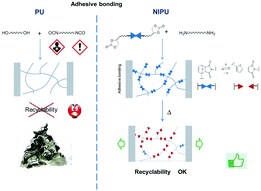
This study describes the synthesis and polymerization of a dicyclocarbonate Diels–Alder (DA) adduct to give a thermoresponsive non-isocyanate polyurethane (NIPU). Firstly a model adduct was synthesized by DA reaction between N-methylmaleimide and furfuryl cyclocarbonate ether (FCE). This adduct was characterized by 1H-NMR and its thermal behavior was studied by 1H-NMR, and differential scanning calorimetry (DSC). Then a telechelic dicyclocarbonate DA adduct was obtained by DA reaction between a bismaleimide oligomer and FCE in bulk with full conversion. Its thermal behavior was studied by thermogravimetric analysis (TGA) and DSC. The dicyclocarbonate adduct was polymerized by step-growth polymerization with a diamine, Jeffamine EDR148. The polymerization was performed at room temperature (in order to avoid adduct deprotection) with triazabicyclodecene as the catalyst. The obtained polymer was characterized by size exclusion chromatography (SEC) and 1H-NMR. The polymer thermal behavior was fully characterized by three complementary analyses. By DSC, retro-Diels–Alder temperatures could be measured to be 90–120 °C. By a 1H-NMR kinetic study at 100 °C, it could be shown that after 120 min at 100 °C, 85% of the adducts are deprotected. Finally, by SEC, it was demonstrated that the obtained NIPU polymer chains undergo thermal scission by rDA reaction.

 Please wait while we load your content...
Please wait while we load your content...