Efficient synthesis and stabilization of poly(propylene carbonate) from delicately designed bifunctional aluminum porphyrin complexes†
Abstract
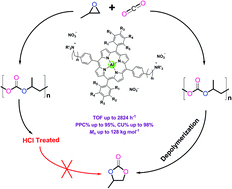
Bifunctional aluminum porphyrin complexes were designed to synthesize poly(propylene carbonate) (PPC) by copolymerization of propylene oxide and carbon dioxide. The catalytic performance is adjustable via delicate control of the electronic environment of the central Al by the number of methoxy groups in the ligand framework as well as the length of the alkyl chain in the quaternary ammonium cation. The optimal catalyst having six methoxy groups in the ligand framework, two trihexylammonium cations linked to benzene via a six-methylene spacer, and NO3− as the axial ligand and quaternary ammonium anions exhibited a TOF of 1320 h−1 at 80 °C and 3 MPa, and a PPC selectivity of 93%, and the TOF even reached 2824 h−1 at 90 °C and 3 MPa, while the PPC selectivity remained at 89%, the highest recorded in aluminum porphyrin complexes to date. In another concern, even though the bifunctional aluminum porphyrin complex has a soil-compostable feature and can be left in PPC without separation, the depolymerization was very rapid even at 25 °C under an ambient atmosphere, and over a 50% decrease in number average molecular weight was observed in 8 days, which could be stabilized by treatment with aqueous HCl solution.

 Please wait while we load your content...
Please wait while we load your content...