Syndioselective ring-opening polymerization and copolymerization of trans-1,4-cyclohexadiene carbonate mediated by achiral metal- and organo-catalysts†
Abstract
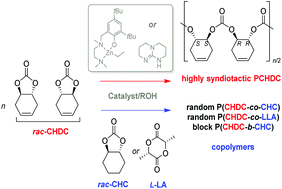
The ring-opening polymerization (ROP) of trans-1,4-cyclohexadiene carbonate (CHDC) has been investigated computationally and experimentally. DFT computations indicate that ring-opening of CHDC is thermodynamically possible, yet to a lesser extent than that of trans-cyclohexene carbonate (CHC). Effective homopolymerizations of rac-CHDC and simultaneous or sequential copolymerizations of rac-CHDC with rac-CHC and L-LA were achieved with a diaminophenolate zinc-based complex ([(NNO)ZnEt]) or a guanidine (TBD) associated with an alcohol. These ROP reactions, which confirmed the lower reactivity of rac-CHDC vs. rac-CHC, especially in homopolymerization, proceeded without any decarboxylation. Quite uniquely, highly syndiotactic PCHDC was obtained from ROP of rac-CHDC with both the zinc- and TBD-based catalysts, as revealed by 13C{1H} NMR studies. The prepared homopolymers and block or random copolymers were characterized by 1H, 13C{1H} NMR, MALDI-ToF MS, SEC and DSC techniques.

 Please wait while we load your content...
Please wait while we load your content...