Synthesis and characterization of branched fullerene-terminated poly(ethylene glycol)s†
Abstract
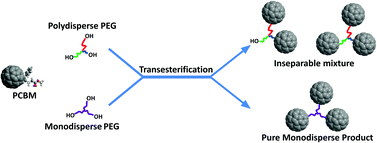
Poly(ethylene glycol) [1, PEG∼4(OH)2, Mn ∼ 200], glycerol ethoxylate [2, PEG∼21(OH)3, Mn ∼ 1000] and pentaerythritol ethoxylate [3, PEG∼15(OH)4, Mn ∼ 797] react directly with phenyl-C61-butyric acid methyl ester (PCBM), in the presence of dibutyltinoxide (DBTO) catalyst at 140 °C, to give a mixture of fullerene [C60] end-capped PEGs via transesterification. Among these PEG linkers, only PEG∼4(OPCB)2 (4a) (OPCB: ester oxygen linked phenyl-C61-butyryl group) was successfully isolated from the crude product mixture in the fully end-capped form. Fully acylated PEG∼21(OPCB)3 (5) and PEG∼15(OPCB)4 (6) could not be separated chromatographically from incompletely reacted species due to the polydispersity in branch lengths. This purification challenge was overcome by using a monodisperse branched core, 1,3,5-tris(octagoloxymethyl)benzene [7, PEG24(OH)3] to give a monodisperse tris-fullerene homostar, PEG24(OPCB)3 (8). The structures of the bis- and tris-fullerene products were confirmed by MALDI-TOF mass spectrometry and 1H NMR spectroscopy with supporting FTIR and UV-vis spectroscopic analysis.

 Please wait while we load your content...
Please wait while we load your content...