A new oligo(hexafluoropropylene oxide)-b-oligo(ethylene oxide) diblock surfactant obtained by radical reactions†
Abstract
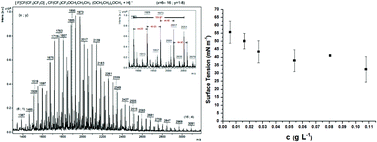
The synthesis and characterization of a new oligo(hexafluoropropylene oxide)-b-oligo(ethylene oxide), oligo(HFPO)-b-oligo(PEG), diblock co-oligomer are presented. First, the model reactions dealing with the radical addition of 1-iodoperfluorohexane (C6F13I) onto allyl alcohol and allyl-O-PEG-OCH3 were optimized in terms of the choice of the initiator (azobisisobutyronitrile [AIBN], tert-butylperoxypivalate [TBBPi], and benzoyl peroxide [BPO]) and of the solvent, temperature, and time. Allyl-O-PEG-OCH3 was obtained from the etherification of ω-hydroxy-PEG with allyl bromide. End-capping of oligo(HFPO) with PEG was successfully achieved by the radical addition of 1-iodoperfluoropropyl-2-oligo(hexafluoropropylene oxide) [oligo(HFPO)-CF(CF3)CF2I] onto allyl-O-PEG-OCH3 using the best conditions of the model reactions. Although TBPPi failed and led to oligo(HFPO)-isobutyl iodide, AIBN and BPO yielded oligo(HFPO)-CH2CHICH2-oligo(PEG). The selective reduction of the latter compound led to oligo(HFPO)-b-oligo(PEG) in 77% yield, the surface tension properties of which were compared to those of commercially available ammonium perfluorooctanoate (APFO) and perfluorooctanoic acid (PFOA). Its critical micelle concentration was 0.04 g mol−1. All models, intermediates, and diblock co-oligomers were characterized by 1H, 19F, and 13C NMR spectroscopy as well as matrix assisted laser desorption ionization (MALDI) and atmospheric pressure solids analysis probe (ASAP) time-of-flight mass spectrometry (TOF-MS).

 Please wait while we load your content...
Please wait while we load your content...