Inverted methoxypyridinium phthalocyanines for PDI of pathogenic bacteria†
Abstract
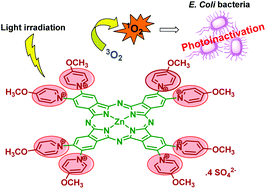
Phthalocyanines (Pc) are photoactive molecules that can absorb and emit light in a large range of the UV-Vis spectrum with recognized potential for medical applications. Considering the biomedical applications an important limitation of these compounds is their low solubility in water. The use of suitable pyridinium groups on Pc is a good strategy to solve this drawback and to make them more effective to photoinactivate Gram-negative bacteria via a photodynamic inactivation (PDI) approach. Herein, an easy synthetic access to obtain inverted tetra- and octa-methoxypyridinium phthalocyanines (compounds 5 and 6) and also their efficiency to photoinactivate a recombinant bioluminescent strain of Escherichia coli is described. The obtained results were compared with the ones obtained when more conventional thiopyridinium phthalocyanines (compounds 7 and 8) were used. This innovative study comparing thiopyridinium and inverted methoxypyridinium moieties on cationic Pc is reported for the first time taking into account the efficiency of singlet oxygen (1O2) generation, water solubility and uptake properties.

 Please wait while we load your content...
Please wait while we load your content...